
Amyotrophic Lateral Sclerosis (ALS)
What is ALS?
Amyotrophic Lateral Sclerosis (ALS) is a rare neurological disorder of progressive deterioration of nerve cells (motor neurons) in the brain and the spinal cord that control muscles throughout the body. When nerve cells die or deteriorate, the brain can no longer control muscle movement, and people with ALS may lose the ability to speak, eat, move and breathe.
The causes of ALS are largely unknown, but inflammation may play a role. Complement is a part of the immune system and helps your body fight infections.
Overactive complement may play a role in ALS through damage to the motor neurons and the activation of inflammation.
4 Areas of Muscle Function Affected by Progression of Amyotrophic Lateral Sclerosis (ALS)
The purpose of this clinical study is to assess the safety and efficacy of ravulizumab-cwvz in adults with ALS. Ravulizumab-cwvz is not currently FDA-approved for the treatment of ALS but is being investigated in the CHAMPION ALS study.
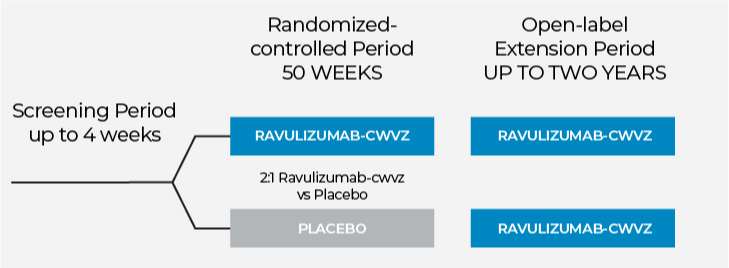
During the Randomized-controlled Period, you will be given the study medication, either ravulizumab-cwvz or placebo, by intravenous infusion (IV) for a total of 50 weeks. Participants can leave a clinical trial at any time and for any reason.
You have a 2 in 3 chance of receiving ravulizumab-cwvz vs. placebo (a substance that looks like ravulizumab-cwvz but does not contain the active ingredient). You will be assigned by chance - like flipping a coin - to receive either ravulizumab-cwvz or placebo. Neither you nor the study coordinators, physicians or nursing staff will know whether you have been given ravulizumab-cwvz or placebo.
Two weeks after the initial dose, you will begin receiving the study medication once every eight weeks. If you are currently taking certain medications for ALS (such as riluzole and/or edaravone) and have not had any recent changes in dosing, you may be able to continue taking them. This will be discussed with you by the study doctors.
After 50 weeks on the study medication, you will have the opportunity to participate in the Open-label Extension Period. In this period, every participant will be given ravulizumab-cwvz, even if they have previously received placebo. The Open-label Extension Period will last for up to 2 years.
Sources:
- 1. NIH Fact Sheet on ALS
- 2. Data on file, Alexion Pharmaceuticals, 2019.
- 3. Parker SE, et al. Revisiting the role of the innate immune complement system in ALS. Neurobiology of Disease. 2019;127:223–232.
*For information on how Alexion collects, uses and protects your personal information, please review our Privacy Notice
Resources for ALS
- The ALS Association
- The Angel Fund for ALS
- ALS One
- ALS Therapy Development Institute
- Clinicaltrials.gov
- Compassionate Care ALS
- I AM ALS
- Les Turner ALS Foundation
- Muscular Dystrophy Association
- NIH: National Institute of Neurological Disorders and Strokes
* The inclusion of this link is not deemed to be an endorsement of or a promotional claim with respect to ravulizumab-cwvz.

