
Myasthenia Gravis (MG)
What is MG?
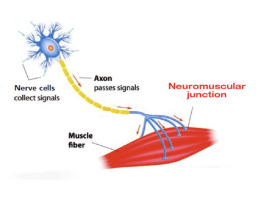
Myasthenia gravis (MG) is a rare autoimmune disorder caused by the immune system attacking the body's own cells. These antibodies activate a pathway in the immune system called the complement pathway that leads to disruption in nerve-to-muscle communication at the neuromuscular junction so that the muscle cannot function normally. People with MG experience significant muscle weakness that can make it difficult to engage in daily activities like brushing their teeth or hair. Although there is no cure for MG, management of the disorder has improved over the past 30 years.
8 ways Myasthenia Gravis (MG) can impact activities of daily living (MG-ADL)
What are some of the study details?
There is a 4 week screening period to determine if the CHAMPION MG study is appropriate for you, which will include blood tests, physical examination and a review of your medical history. If you are eligible to participate, you will either receive ravulizumab-cwvz or placebo (a medication that looks the same as the investigational drug, but contains no active ingredient). You will be given the study drug (either ravulizumab-cwvz or placebo) at the start and then 2 weeks later, followed by treatments every 8 weeks for a total of 26 weeks.

You have a 50% chance of receiving ravulizumab-cwvz, which will be given as an infusion through a vein at the study site.
During this time you will be asked for your feedback on your symptoms and quality of life. After 26 weeks on the study drug, you will be given the opportunity to enroll in the extension period of the study and will be given ravulizumab-cwvz, even if you had received placebo during the first study treatment period. The extension period can last up to 2 years, or until the drug is approved for MG.
You can withdraw from the study at any time, and the costs of study visits and study medications will be paid by the study's sponsor. There may also be assistance for travel if there is no study site in your immediate area.
Sources:
- Conte-Fine B, et al. J Clin Invest. 2006; 116(11):2843-2857
- Fang, F, Sveinsson, O, Thormar, G, Granqvist, M, Askling, J, Lundberg, IE, Piehl, F. 2015. The autoimmune spectrum of myasthenia gravis: a Swedish population-based study. J Intern Med, 277(5), 594-604. doi:10.1111/joim.12310
- Muppidi S, Wolfe G, Conaway M, et al. MG-ADL: Still a relevant outcome measure. Muscle Nerve2011; 44: 727–731.
- http://www.clinicaltrials.gov/ct2/show/NCT03920293
- NIH Myasthenia Gravis Fact Sheet https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Myasthenia-Gravis-Fact-Sheet#3
- Data on file, Alexion Pharmaceuticals, 2019.
*For information on how Alexion collects, uses and protects your personal information, please review our Privacy Notice

